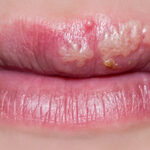
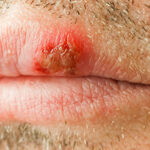
What Are Cold Sores?
Cold sores, sometimes called fever blisters, are small blisters that appear periodically.¹ These blisters can itch and burn, and can be especially painful when they break open. Cold sores are also contagious, and can take up to two weeks to heal.
Where do cold sores commonly form?
Cold sore outbreaks typically occur on the face and lips, which is arguably the worst thing about them.¹ After all, who wants to sit through class, give a presentation, or go on a date with a cold sore on their lip? For this reason, cold sore sufferers often look for treatment options to help minimize the number of outbreaks they have to deal with, and/or to shorten the healing process.
Additional resources:
- WedMD (June 04, 2014). Cold Sores – Topic Overview [Skin Problems & Treatments Health Center web post]. Retrieved August 15, 2015, from http://www.webmd.com/skin-problems-and-treatments/tc/cold-sores-topic-overview
Indication & Important Safety Information
INDICATION
Sitavig® (acyclovir) 50 mg buccal tablet is indicated for
the treatment of recurrent herpes labialis (cold sores) in immunocompetent
adults.
IMPORTANT SAFETY INFORMATION
- Sitavig® (acyclovir) 50 mg buccal tablet should not be used in patients with known hypersensitivity to acyclovir, milk protein concentrate, or any other component of the product.
- Sitavig has not been studied in immunocompromised patients. No interaction studies have been performed. Sitavig’s safety and efficacy have not been established in pediatric patients.
- There are no available data on Sitavig use in pregnant women. However, published observational studies over decades of use of acyclovir have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. It is not known if Sitavig is excreted in breast milk; however, systemic exposure following buccal administration of acyclovir is minimal. Before administration, discuss if the patient is lactating or planning to breastfeed.
- The possibility of viral resistance to acyclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
- In a controlled clinical trial, the most common side effects (greater than or equal to 1%) for Sitavig were: headache (3%), dizziness (1%), lethargy (1%), gingival (gum) pain (1%), aphthous stomatitis (canker
sores) (1%), application site pain (1%), application site irritation (1%), erythema (redness) (1%), and rash (1%). In the same trial, these side effects ranged from 0% to 3% for placebo.
You are encouraged to report negative side effects of prescription
drugs to the FDA. Call
1-800-FDA-1088 or visit www.fda.gov/medwatch.
Please see Full Prescribing Information.