The Science Behind Sitavig
The convenient, discrete choice.
Benefits of Sitavig:
- No pill to swallow
- Invisible to others
- Ideal for cosmetic- and appearance-conscious patients with recurrent herpes labialis
- Treatment does not interfere with most daily life activities
- One-time dose
- Second tablet provided in every prescription as backup or for a future outbreak
- Patients generally know their triggers, and should keep Sitavig available to them especially during these times
Where HSV-1 replication takes place
- HSV-1 begins to replicate in the basal layer of the oral mucosa.
- Patient may experience an itching, burning or tingling sensation.
- A lesion may form in 2-3 days.

Lauriad™ delivery system¹
- Muco-Adhesive Buccal Tablet applied to the upper gum over the canine fossa
- Targeted delivery of antiviral drug directly to the site of viral replication
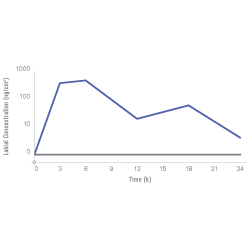
Stays active during viral replication¹
- Specifically designed to slowly dissolve over 12-14 hours
- Covers the window of maximum viral replication
- Provides continuous, targeted delivery of acyclovir
Sitavig reduces salivary viral load2
A single dose of Sitavig reduced the viral load in the saliva 5-fold at the site of viral replication (P=.583), possibly reducing the amount of virus available to return into latency.

More days between outbreaks3*
Patients treated with Sitavig had a average of 105 more days before experiencing a recurrent episode of herpes labialis compared to patients treated with placebo. Plus, 36% of patients remained free from lesion recurrence at 10 months post-treatment compared to 26% of placebo patients (P=.027).
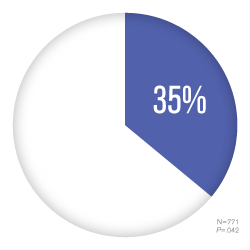
Sitavig patients experienced 35% aborted episodes3
34.9% of patients failed to develop a vesicular lesion when applying Sitavig soon after feeling an oncoming cold sore sensation.
86% of patients were satisfied with Sitavig treatment²
*Data on the incidence and delay of the next recurrence need further confirmation as they were obtained on a subset of patients.
References
- Lemarchand C, Singlas E, Constantini D, Dufour A and Attali P. Plasma, saliva and labial mucosa pharmacokinetics of acyclovir Lauriad mucoadhesive buccal tablet in healthy volunteers. J Clin Pharmacol Clin Pharmacokinet. 2014;1(1):1-9.
- Data on file. EPI Health.
- Bieber T, Chosidow O, Bodsworth N, Tyring S, Hercogova J, Bloch M, Davis M, Lewis M, Boutolleau D, Attali P and the LIP Study Group. Efficacy and safety of acyclovir mucoadhesive buccal tablet in immunocompetent patients with labial herpes (LIP): A double-blind, placebo-controlled, self-initiated trial. J Drugs Dermatol. 2014;13(7):791-798. View study (link will lead you to the JDD site).
Indication & Important Safety Information
INDICATION
Sitavig® (acyclovir), 50mg Muco-Adhesive Buccal Tablet is indicated for
the treatment of recurrent herpes labialis (cold sores) in immunocompetent
adults.
IMPORTANT SAFETY INFORMATION
- Sitavig should not be used in patients with known hypersensitivity to acyclovir, milk protein concentrate, or other components of the product.
- Sitavig has not been studied in pregnant women or in immunocompromised patients and no interaction studies have been performed. Sitavig’s safety and efficacy have not been established in pediatric patients.
- Sitavig is a Pregnancy Category B product; therefore it should be used during pregnancy only if the potential benefit outweighs the potential risk to the fetus. It is not known if Sitavig is excreted in breast milk; however, systemic absorption is minimal.
- In a controlled clinical trial Sitavig’s most common side effects (greater than or equal to 1%) were: headache (3%), dizziness (1%), lethargy (1%), gingival (gum) pain (1%), aphthous stomatitis (canker
sores) (1%), application site pain (1%), application site irritation (1%), erythema (1%) and rash (1%). In the same trial these side effects ranged from 0%-3% for placebo.
You are encouraged to report negative side effects of prescription
drugs to the FDA. Visit www.fda.gov/medwatch.com or call
1-800-FDA-1088. Click here for Full Prescribing Information.

